Previous Pilot Project Winners
Year 1 Winning Proposals
Pulmonary and immuno-toxicological effects of exposure to metals released by e-cigarettes and other emerging ultra-portable electronic nicotine delivery systems
Thivanka Muthumalage, Ph.D.
Determining the Appeal and Abuse Liability of Heated Tobacco Products: Qualitative and Behavioral Economic Evaluation of the Role of Flavor
Amanda Quisenberry, Ph.D.
Year 2 Winning Proposals
Impact of nicotine salts as contained in Juul on pulmonary damage using in vitro and in vivo models
Tariq Bhat, Ph.D.
Prenatal Vaping in the United States: Patterns of E-cigarette Use and Cessation by Product Characteristics and Maternal Demographics
Elaine Hill, Ph.D.
Year 3 Winning Proposals
Developing engineered tissue platform for screening the pro-fibrotic effects of flavored e-cigarettes
Qixin Wang, Ph.D.
Preventing e-Cigarette Use among Latino and African American Adolescents One Message at a Time
Francisco Cartujano-Barrera, MD
Year 4 Winning Proposals
Effect of use of different doses and flavors of e-cig aerosols towards health effect and anti-viral responses in COVID-19
Gagandeep Kaur, Ph.D.
Mixed methods approach to assess Tobacco 21’s impact on youth and early young adult tobacco use behaviors
Liane Schneller, Ph.D.
Application Deadline: 2 May 2022
Project Start Date: 1 September 2022
Description: The aim of this RFA is to support new and innovative mentored pilot research relevant to the regulation of tobacco products by the Food and Drug Administration (FDA) Center for Tobacco Products (CTP). One goal of the pilot grants program is to foster careers in tobacco regulatory science-relevant research. Funded projects should lead to development of an application for externally funded research, for example, through the Tobacco Regulatory Science Program at the NIH and FDA (https://prevention.nih.gov/tobacco-regulatory-research), or through other NIH Institutes or Centers.
Projects will be required to identify a clear tobacco regulatory science (TRS) goal aligned with one or more FDA CTP priorities. Priority areas are: Toxicity, Addiction, Health Effects, Behavior, Communications, Marketing Influences, and Impact Analysis, all related to tobacco products including electronic nicotine delivery systems (ENDS). Additional detail on these priority areas can be found at https://www.fda.gov/TobaccoProducts/PublicHealthScienceResearch/Research/ucm311860.htm. The NIH and FDA encourage use of the PhenX Toolkit (www.phenxtoolkit.org) and other common data elements (https://www.nlm.nih.gov/cde/), as appropriate. Priority will be given to projects that include collaborators from both Roswell Park and the University of Rochester and that engage with CRoFT research projects and cores as appropriate (https://www.flavoredtobacco.org/). For questions about specific CRoFT projects and cores, please click on “Contact” at the bottom of each page on this CRoFT flavored tobacco website, or email croft@roswellpark.org.
Following Notifications of Award, Graduate students, Postdoctoral Fellows and New/Early Stage and TRS transitioning applicants will identify a mentoring team to include at least one CRoFT investigator; will consult with the Biostatistics & Informatics Core to ensure appropriate study design, power calculation, and analysis plan; and will create a TRS career enhancement plan. This plan will use existing resources at both institutions, such as coursework from existing certificate programs; elective coursework including audits; an internship/rotation; TRS seminars and colloquia; Un-Meetings; participation in synergy manuscripts, and/or other team science experiences. Pilot awardees will present their work at the annual CROFT conference, held alternately at Roswell Park and the University of Rochester.
Eligibility: Mentored pilot projects are open to full-time researchers at Roswell Park or the University of Rochester in any of the following categories: Graduate student with at least one year of research experience and two years left in graduate school, Postdoctoral Fellows with at least 1.5 years remaining on their funded training period at the time of award, New/Early Stage investigators, investigators transitioning to TRS, and current TRS investigators exploring new areas of TRS research. No funding from tobacco or e-cigarette industries in past 5 years is allowed (see https://www.drugabuse.gov/research/clinical-research/regulations-policies-guidance/points-to-consider- regarding-tobacco-industry-funding-nida-applicants). Each applicant may submit only one proposal as PI or MPI, though they may be listed as co-investigators on other submissions. Previous applicants are eligible to submit a project with new aims and different collaborators.
Funding: This RFA will fund up to 2 meritorious mentored pilot projects. A maximum of $13,600 each will be awarded for a 12-month period. Funding will include project costs, travel to a scientific meeting for presentation of project results, and travel to other TCORS or related sites for a rotation and/or collaboration.
Deadlines:
- 2 May 2022 at 11:59 PM – Full proposals must be received Proposals received after 11:59 PM will be rejected.
- 1 August 2022 – Notifications of Award will be sent out by August 1, 2020
- 1 September 2022 – Anticipated start date
Application Guidelines: The application should be submitted through the REDCap portal at https://redcap.link/pilot_yr5. We encourage you to contact a CRoFT investigator prior to submitting to discuss your idea. A 3-page research plan, including all tables and figures (but not including references, budget, budget justification, and biosketches), should be uploaded and include the following components:
- Single-spaced, 11-point font minimum, at least 0.5-inch wide margins.
- The following Research Plan sections: specific aims, background and significance, innovation, research design and methods, future research plans.
- Clearly identify how the proposed pilot addresses one or more of the FDA TRS priority areas:
https://www.fda.gov/TobaccoProducts/PublicHealthScienceResearch/Research/ucm311860.htm
- Grant Potential – clear description of how a successful pilot project will lead to a subsequent grant submission (e.g., F31/F32/K99/K01 for pre-doc, F32/K99/K01/R21/R01 for post-doc, R01 for faculty).
- Clearly identify any collaborations between Roswell Park and the University of Rochester, and/or with other TCORS investigators.
- Include an NIH biosketch for yourself and other key investigators.
- For pre-doctoral students and postdoctoral fellows, include a letter of support from your current mentor, who should also agree to serve on your mentoring team, and from a proposed primary mentor for your proposed project if that person is different from your current mentor.
Budget and Justification: Include a budget (in NIH format) and budget justification. Funds can only be used for direct costs associated with project research activities, staff or student support to implement project, project-related travel for conference travel and travel to other TCORS or related sites for rotations and/or collaborations to enhance TRS research experience, and purchase of research materials including data sets. Investigator salary is not allowed.
If Funded – Requirements Prior to Release of Funds:
- Proof of IRB or IACUC approval, as appropriate. For projects with an MPI at Roswell Park and U of Rochester, a central IRB through Roswell will be used in lieu of the local IRB.
- Proof of Delayed Onset approval from NIH: The NIH requires that CRoFT obtain explicit approval from the NIH for any pilot-funded research involving human subjects. Accordingly, the IRB-approved protocol and other materials must be submitted to the NIH at least 30 days prior to the project start date. CRoFT personnel will work with awardees to meet these requirements.
- Proof of Prior Approval of Vertebrate Animals Research: The NIH requires that CRoFT obtain explicit approval from the NIH for any pilot-funded research involving vertebrate animals. IACUC approval documentation and other materials must be submitted to the NIH at least 30 days prior to the project start date. CRoFT personnel will work with awardees to meet these requirements.
Reporting Requirements: Awardees will submit a mid-year report at the 6-month point following funding. Within 60 days after the end of the project, awardees will submit a final written report. Applicants will present their research at the annual CRoFT conference in Buffalo or Rochester, depending on year, as described above. Applicants will agree to participate in brief follow-up surveys of TRS activities in the years following funding.
Publications: All publications that benefit in whole or in part from support provided by CRoFT must:
- Comply with the NIH Public Access Policy: Information regarding the Public Access Policy is located on the University of Rochester Miner Library website at http://www.urmc.rochester.edu/libraries/miner/publishing/NIHPublicAccessPolicyMinerLibrary.cfm.
- Acknowledge CRoFT grant funding as follows:
- “Research reported in this [publication/press release/etc.] was supported by the National Cancer Institute of the National Institutes of Health (NIH) and the Food and Drug Administration (FDA) Center for Tobacco Products under Award Number U54CA228110. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA.”
- Prior to issuing a press release concerning the outcome of this research, please coordinate with your department’s communication contact person and notify Craig Steger, MA, Steger@RoswellPark.org , who will then notify the National Cancer Institute in advance to allow for coordination.
Review Priorities: Priorities for awarding pilot funding include:
- Responsiveness to the RFA and FDA priorities.
- Quality of the proposed science.
- Potential to stimulate subsequent independent funding.
- Collaborations. Proposals that include collaboration between U of Rochester and Roswell Park are preferred.
Note for pre-doctoral applicants: Review of proposals submitted by pre-doctoral students will particularly consider the following: research experience and training, evidence of research productivity, potential to become an independent investigator in the area of tobacco regulatory science research.
Review Process: Proposals will be reviewed by the CRoFT Pilot Review Committee, consisting of investigators from Roswell Park and the University of Rochester, and other selected ad hoc experts as needed to allow for rigorous scientific review. Decisions will be made following review, and a formal study section-style discussion and scoring meeting. Trainee proposals will be reviewed separately from the other categories.
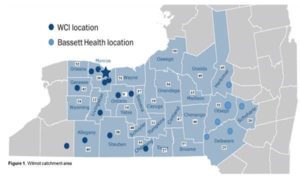
Additional Funding Opportunity for University of Rochester Applicants: To further CRoFT’s mission in helping to inform the FDA CTP regarding the regulation of tobacco products, optional supplemental funding is available to University of Rochester pilot project applicants to be used within the 12-month period of the parent award (specified above). Applicants have the option to include a request for supplemental funding of up to $13,000 if the mentored pilot project addresses existing cancer disparities focused on e-cigarettes and/or vaping flavors. The project must also include a community outreach and engagement component, with at least 50% of pilot project supplement participants residing in the catchment area (see below). Please refer to the National Cancer Institute https://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-089.html for the definition of disparity groups. Rural-based projects are particularly encouraged.
This optional $13,000 supplemental funding will be in addition to the $13,600 available to all pilot project applicants. Therefore, projects must align with one or more FDA CTP priorities with the added disparities and community outreach component. A request for supplemental funding is not required for pilot project applications, but all supplemental funding requests must stem from a parent CRoFT pilot project proposal.
To apply for this supplemental award please provide an additional budget justification and an additional 1-page narrative describing your community outreach and engagement proposal and how it builds on your mentored pilot project proposal. The REDCap submission template will include sections for these optional supplemental requests for applicants who wish to include this component.
Contact
Application Contact:
Jacqueline Attia, MPH WNY_CRoFT@urmc.rochester.edu
Scientific/Research Contact:
Scott Steele, PhD scott_steele@urmc.rochester.edu
Deborah Ossip, PhD deborah_ossip@urmc.rochester.edu